Biosimilar Interchangeability Checker
Search for Biologic Medication
How This Works
This tool checks if there are FDA-approved interchangeable biosimilars for the medication you're looking for, and whether your state allows automatic substitution without a new prescription.
The Purple Book is the U.S. Food and Drug Administration’s official database for biological products, including biosimilars and interchangeable versions. It’s not a book you can hold - it’s a live, searchable tool on FDA’s website that tells you exactly which biological medicines are approved, which ones are biosimilars, and which ones can be swapped out by pharmacists without needing a new prescription. For patients, pharmacists, and doctors, this resource answers the most pressing questions: Can I switch from the brand-name drug to a cheaper version? Is that switch safe? Who decides?
What Is the Purple Book, Really?
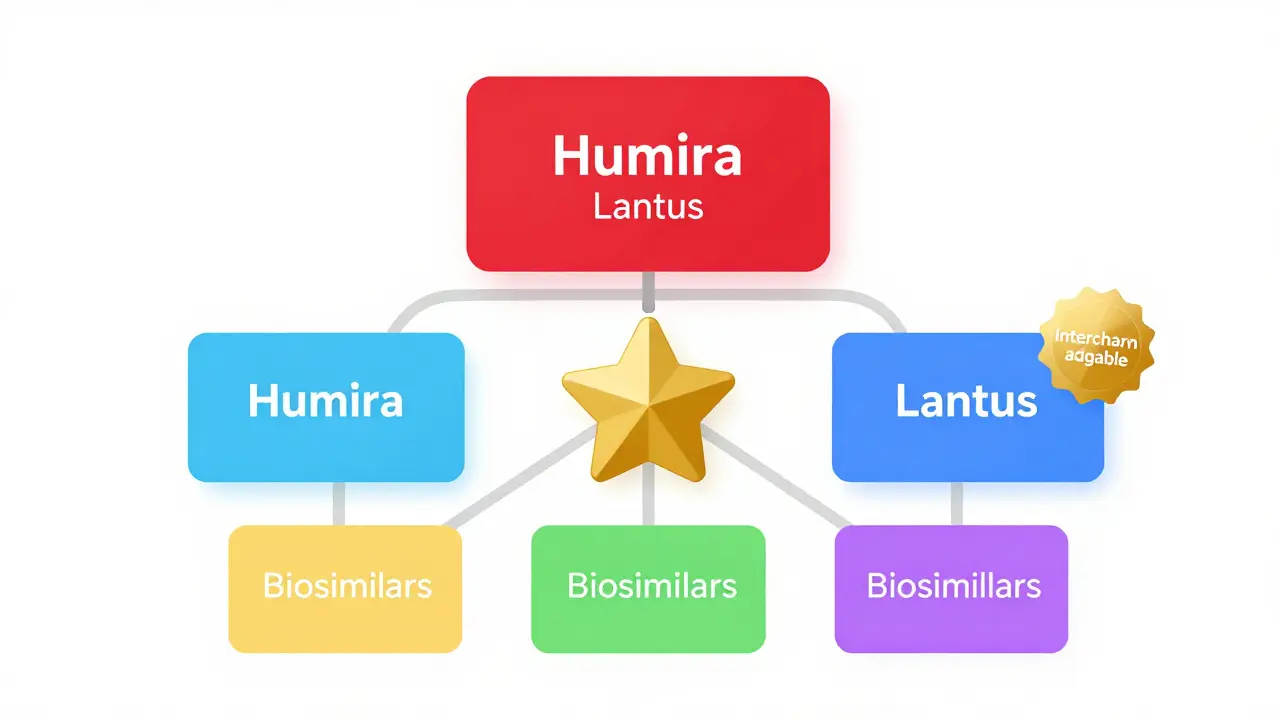
The Purple Book started as two separate lists - one for drugs managed by the Center for Drug Evaluation and Research (CDER), and another for biologics handled by the Center for Biologics Evaluation and Research (CBER). In 2020, the FDA merged them into one searchable database. Now, it includes everything from insulin and rheumatoid arthritis treatments to vaccines, cell therapies, and blood products. Each entry shows the product’s approval date, whether it’s a reference product (the original), a biosimilar, or an interchangeable version.Think of it like a family tree. At the top is the original biologic - the reference product. Below it, you’ll find its biosimilar relatives. Some of those relatives are marked as interchangeable, meaning they’ve passed an extra level of testing. The database uses color-coded cards to visually group related products. If two cards are the same color, they’re linked - one is the original, the other is a copy that’s been approved as similar or interchangeable.
Biosimilars vs. Interchangeable: What’s the Difference?
All interchangeable products are biosimilars, but not all biosimilars are interchangeable. That’s the key point most people miss.A biosimilar is a biological product that’s highly similar to the original - no clinically meaningful differences in safety, purity, or strength. The FDA requires extensive lab testing and clinical trials to prove this. But even after approval, a biosimilar can’t be automatically swapped by a pharmacist unless it’s also designated as interchangeable.
An interchangeable product has to meet one more requirement: it must show that switching back and forth between it and the original drug doesn’t increase risk or reduce effectiveness. That means if a patient gets the brand-name drug one month, then switches to the biosimilar the next, and then back again, their body responds the same way every time. This is proven through specific switching studies - not just one-time comparisons.
The FDA is clear: interchangeability doesn’t mean the product is better. It just means it’s safe to swap. A biosimilar that isn’t interchangeable works just as well - it just can’t be substituted without the prescriber’s permission.
Why Does Interchangeability Matter?
The real impact of interchangeability happens at the pharmacy counter. In most states, pharmacists can swap a prescribed biologic for an interchangeable biosimilar without calling the doctor. That’s a big deal because biologics can cost tens of thousands of dollars a year. A biosimilar might save a patient $5,000 or more annually. But if it’s not interchangeable, the pharmacist has to check with the doctor first - delaying care and adding paperwork.As of late 2023, only seven biosimilars had received the interchangeable designation from the FDA. These include two insulin products, three for inflammatory conditions like Crohn’s disease and psoriasis, and two for eye conditions. More are in the pipeline. Companies are actively submitting applications, knowing that interchangeability opens the door to broader use.
But here’s the catch: federal approval doesn’t guarantee automatic substitution. Each state has its own rules. As of 2023, 47 states and Puerto Rico allow pharmacists to substitute interchangeable biosimilars without prescriber approval. But even in those states, pharmacists may still need to notify the prescribing doctor, document the switch, or inform the patient. A few states require the patient to consent before substitution. This patchwork of laws makes it harder for patients and providers to know what’s allowed where.

What’s in the Purple Book? Real Examples
Search for Humira in the Purple Book, and you’ll see its reference product card. Below it, you’ll find several biosimilars - Adalimumab-atto, adalimumab-adbm, adalimumab-afzb. One of them, adalimumab-afzb, is marked as interchangeable. That means in states that allow substitution, a pharmacist can give you that version instead of Humira without asking your doctor.Same with insulin. Insulin glargine (Lantus) has two interchangeable biosimilars: Semglee and Rezvoglar. Both are approved as interchangeable, so if your prescription says “insulin glargine,” your pharmacist can fill it with either of those - and in most states, they don’t need to check with your doctor first.
Each product card also shows icons for delivery methods - autoinjector, pre-filled syringe, vial - so you know what form the medicine comes in. That’s helpful if you’re used to a certain device and want to make sure the biosimilar works the same way.
What the Purple Book Doesn’t Tell You
The Purple Book doesn’t list prices. It doesn’t say which biosimilars are covered by your insurance. It doesn’t explain state laws in detail. And it doesn’t tell you if a product is branded or unbranded - even though the FDA notes that “unbranded biologics” are considered equivalent to brand-name products, they’re not the same as interchangeable biosimilars.It also doesn’t include products that are still under review. If you’re looking for a biosimilar that just got submitted, you won’t find it until the FDA approves it. The database is updated regularly, but not in real time.
How to Use the Purple Book
Go to the FDA’s website and search for “Purple Book.” You’ll land on the searchable database. Start by typing the brand name of the biologic you’re interested in - like Enbrel, Remicade, or Neulasta. The results will show the reference product and all its biosimilar and interchangeable copies. Click on any product to see its full profile: approval date, exclusivity status, and designation.If you’re a pharmacist, use the color-coded cards to quickly identify which products can be substituted. If you’re a patient, use it to confirm whether your pharmacy is offering a biosimilar that’s been approved for interchange. If you’re a provider, use it to understand what alternatives are legally available for your patients.
There’s a filter option to show only interchangeable products. That’s the fastest way to find the ones that can be swapped without a new prescription.
What’s Next for Biosimilars?
The FDA continues to release guidance on labeling, manufacturing, and clinical studies for biosimilars. In 2023, they published draft guidance on how biosimilar labels should be written - to avoid confusion and ensure patients and providers know exactly what they’re getting.More companies are investing in interchangeability studies because the payoff is big. Once a biosimilar gets the interchangeable label, it can compete directly with the original - not just on price, but on ease of use. Pharmacists can make the switch automatically. Insurance companies may favor interchangeable products in their formularies. Patients get lower costs without extra steps.
But progress is slow. The science is complex. The regulatory path is long. And state laws still vary. The Purple Book doesn’t solve all the barriers - but it’s the clearest, most reliable source of truth about what’s approved and what’s interchangeable.
For anyone managing chronic conditions with biologics - whether it’s rheumatoid arthritis, diabetes, or Crohn’s disease - knowing how to use the Purple Book means knowing your options. It means asking your pharmacist: “Is there an interchangeable biosimilar for this?” And if they say no, asking why. Because the answer might be in the database - and you’re the one who can find it.
What is the Purple Book?
The Purple Book is an online database maintained by the U.S. Food and Drug Administration (FDA) that lists all FDA-approved biological products, including biosimilars and interchangeable versions. It helps patients, pharmacists, and doctors identify which products are similar to or can be substituted for brand-name biologics.
What’s the difference between a biosimilar and an interchangeable product?
A biosimilar is a biological product that’s highly similar to the original (reference) product with no clinically meaningful differences in safety or effectiveness. An interchangeable product is a type of biosimilar that has passed additional testing to prove it can be safely switched back and forth with the original product without increasing risk or reducing effectiveness. All interchangeable products are biosimilars, but not all biosimilars are interchangeable.
Can a pharmacist automatically substitute an interchangeable biosimilar?
In most states, yes - but only if the product is designated as interchangeable by the FDA and state law allows substitution. As of 2023, 47 states and Puerto Rico permit pharmacists to substitute interchangeable biosimilars without a new prescription. However, some states require the pharmacist to notify the prescriber, inform the patient, or document the change.
Does the Purple Book list prices for biosimilars?
No. The Purple Book provides regulatory status - like whether a product is biosimilar or interchangeable - but it does not include pricing, insurance coverage, or availability information. Patients should check with their pharmacy or insurer for cost details.
How many interchangeable biosimilars are approved as of 2025?
As of late 2023, the FDA had approved seven interchangeable biosimilars, including two insulin products, three for inflammatory diseases, and two for eye conditions. More are under review, and the number is expected to grow in 2025 as companies submit additional applications.
Is the Purple Book updated regularly?
Yes. The FDA updates the Purple Book regularly as new products are approved or designations change. It’s a live database, not a static document. Users should check the official FDA website for the most current information.
People think this is progress? It's corporate greed wrapped in regulatory jargon. And don't even get me started on how pharmacies profit from substitution without patient consent.
Importantly, the FDA’s designation of interchangeability is predicated upon a rigorous, multi-tiered analytical framework-requiring not only analytical similarity, but also clinical switching studies demonstrating no increased risk of adverse events or diminished efficacy upon multiple switches. This is not a trivial distinction.
I don’t care if it’s branded or unbranded-I care that they get the drug they need, on time, without bankruptcy.