When you take a medication like levothyroxine or tacrolimus, you might assume all generic versions are the same. But for drugs with a narrow therapeutic index (NTI), even tiny differences between manufacturers can matter - a lot.
What Makes a Drug an NTI Drug?
NTI drugs have a razor-thin line between working and causing harm. A small change in blood levels - even 5% - can mean the difference between controlling your seizures, keeping your transplant alive, or landing in the hospital. These aren’t your typical painkillers or antibiotics. They’re the kind of meds where precision is non-negotiable.
The FDA doesn’t publish a full list, but they’ve flagged key ones: warfarin, digoxin, lithium, phenytoin, carbamazepine, cyclosporine, tacrolimus, and theophylline. For these, the safe dose range is so narrow that the therapeutic index - the ratio between a toxic dose and an effective dose - is often just 2 to 4. That means if your body absorbs 10% more of the drug, you could be in danger.
How Are Generic NTI Drugs Tested?
Generic drugs must prove they’re bioequivalent to the brand-name version. For most drugs, that means the generic’s blood concentration must fall within 80-125% of the original. But for NTI drugs, the FDA tightens the rules. Starting in 2018, they began requiring tighter limits - often 90-111% or even 95-105% for the active ingredient itself.
Why? Because standard testing doesn’t always catch what happens in real people. A 2022 FDA review found that while average differences between generics and brand-name NTI drugs were small - around 3.5% for overall exposure (AUC) and 4.3% for peak levels (Cmax) - individual patients didn’t always respond the same way. One person might absorb a generic version perfectly. Another might have wild swings in blood levels, even if the average looks fine.
Real-World Problems: When Switching Goes Wrong
Studies show switching between generic versions of NTI drugs can cause real trouble - even when each version passes FDA tests.
In kidney transplant patients, switching from one formulation of cyclosporine to another led to a 15.3% higher rate of organ rejection. For tacrolimus, one study found blood concentration variability jumped to 21.9% after switching manufacturers. That’s not a typo. Nearly one in five patients had unpredictable levels. Some went too low - risking rejection. Others went too high - risking kidney damage or nerve problems.
For warfarin, a 2019 study found switching between generic manufacturers increased INR variability by 0.32 points - small on paper, but meaningful in practice. INR needs to stay between 2 and 3 for most patients. A shift of 0.3 could mean the difference between clotting and bleeding.
And it’s not just transplant or blood-thinner patients. A 2019 survey of pharmacists found that 63% had received complaints from patients or doctors after switching generic NTI drugs. People reported new seizures, mood swings, fatigue, or unexplained lab changes.
Why Do These Differences Happen?
It’s not about the active ingredient. All generics contain the same chemical. The problem lies in the fillers, coatings, manufacturing processes, and how the drug dissolves in your gut.
Take tacrolimus. One study compared four generic brands to the original. The active ingredient ranged from 86% to 120% of the labeled amount. That’s a huge spread. Even though none broke FDA rules, the differences were enough to affect absorption in sensitive patients.
Some generics use different binders or coatings that change how quickly the pill breaks down. For a drug like levothyroxine, which needs consistent absorption to keep your thyroid levels stable, even a 5% variation in absorption can throw off your TSH. That’s why some doctors still prefer brand-name Synthroid - not because generics are unsafe, but because consistency matters more than cost.
What Do the Experts Say?
The FDA says generic NTI drugs are therapeutically equivalent. And for most people, they are. A 2021 FDA analysis of over 10,000 patients switching from brand-name to generic levothyroxine found no meaningful difference in TSH levels. The average was 2.12 vs. 2.15 - statistically identical.
But here’s the catch: population averages don’t tell the whole story. A 2022 review in the Journal of Clinical Pharmacology pointed out that while most patients do fine, a small subset - maybe 5-10% - are extremely sensitive. For them, switching manufacturers can be disruptive.
That’s why medical societies like the American Academy of Neurology recommend against automatic substitution for antiepileptic NTI drugs like phenytoin and carbamazepine. They’ve seen patients lose seizure control after switching, even when labs looked normal.
Meanwhile, the American Medical Association noted in 2007 that brand-name drug companies change their formulations too - sometimes without telling anyone. So if you’re worried about generics, you should also be aware that brand-name versions aren’t always the same over time.
What Should You Do?
If you’re on an NTI drug, here’s what actually works:
- Stick with one manufacturer - if your prescription works, don’t switch unless you have to. Ask your pharmacist to keep you on the same generic brand.
- Know your drug’s name - if your pill looks different, ask why. Generic names aren’t always printed clearly. Write down the manufacturer (e.g., “Mylan,” “Accord,” “Sandoz”) and ask for the same one next refill.
- Monitor closely - if you’re on warfarin, check your INR after any switch. If you’re on levothyroxine, get your TSH tested 6-8 weeks after a change. For tacrolimus or cyclosporine, your doctor should check blood levels more often.
- Speak up - if you feel different after a switch - more tired, more anxious, new symptoms - tell your doctor. Don’t assume it’s “just in your head.”
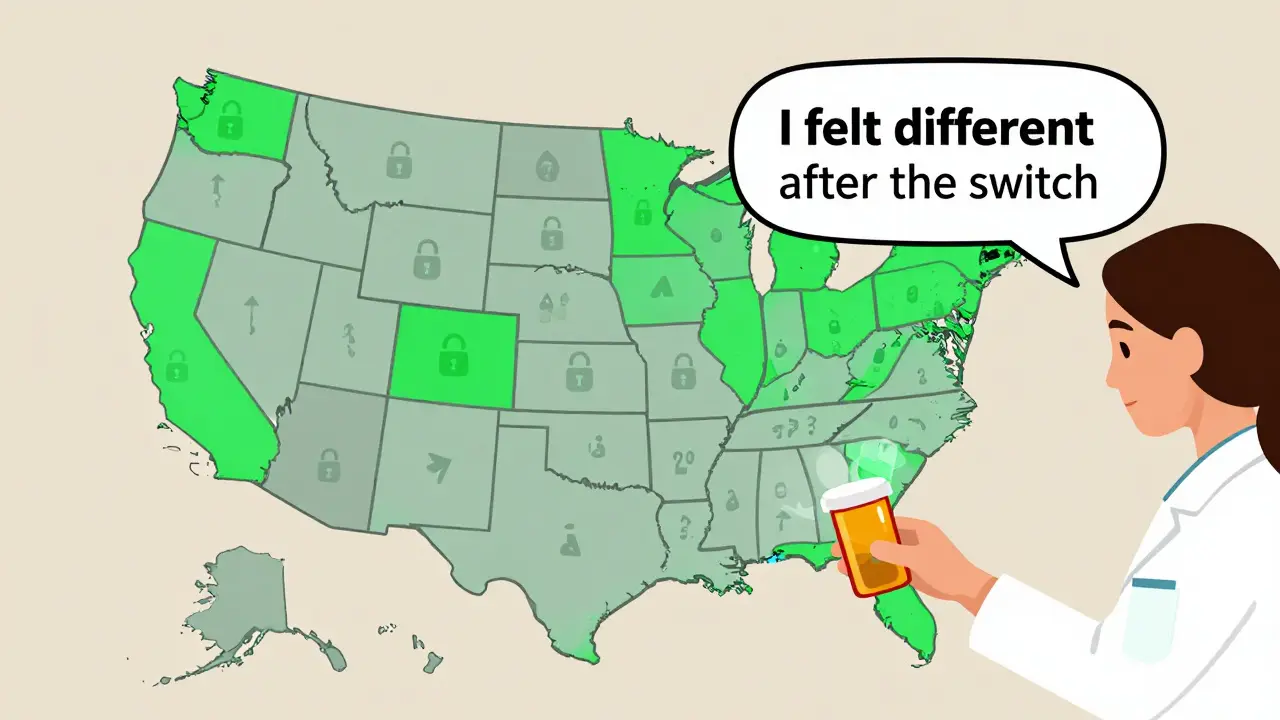
- Know your state’s laws - 27 states have laws restricting automatic substitution for NTI drugs. In some places, pharmacists must get your doctor’s OK before switching. Ask if your state protects you.
Bottom Line: It’s Not Black and White
Generic NTI drugs are safe for most people. The FDA’s standards are strict, and the data shows that, overall, they work. But for a small group - those with high sensitivity, complex conditions, or unstable health - switching between manufacturers can be risky.
There’s no perfect solution. The system balances cost and safety. But if you’re on one of these drugs, you’re not just a statistic. Your body reacts uniquely. Your stability matters more than a pharmacy’s inventory.
Don’t let cost savings override your health. If you’re doing well on a specific brand or generic, keep it. If you’re switched without warning, track your symptoms. And always, always talk to your doctor before accepting a change.
Are all generic NTI drugs the same?
No. While all generics contain the same active ingredient, differences in inactive ingredients, manufacturing methods, and how the drug dissolves can lead to variations in absorption. For NTI drugs, even small differences can affect blood levels and clinical outcomes.
Can I switch between generic NTI drugs safely?
For most people, yes - but not without caution. If you’re stable on one generic, avoid switching unless necessary. If you must switch, monitor closely. For drugs like warfarin, levothyroxine, or tacrolimus, get lab tests 4-8 weeks after the switch to ensure your levels haven’t shifted.
Why do some doctors prefer brand-name NTI drugs?
Some doctors prefer brand-name versions because they’ve seen patients experience instability after switching generics - especially with antiepileptics or immunosuppressants. While studies show generics are generally equivalent, individual variability means some patients are more sensitive. Consistency matters more than cost for these cases.
Is levothyroxine safe as a generic?
Yes, for the vast majority of patients. A 2021 FDA analysis of over 10,000 patients found no significant difference in TSH levels between brand-name Synthroid and generic levothyroxine. However, a small number of patients report symptoms after switching, so consistent use of the same manufacturer is recommended.
What should I do if I think my generic NTI drug isn’t working?
Don’t stop taking it. Contact your doctor immediately. Keep track of symptoms, when they started, and whether you recently switched manufacturers. Your doctor may order a blood test (like INR for warfarin or TSH for levothyroxine) and consider switching you back to your previous version. Never assume it’s “just anxiety” - your concerns are valid.
Do all states restrict switching NTI drugs?
No. As of 2022, 27 states have laws that limit or require permission for automatic substitution of NTI drugs. In those states, pharmacists must get your doctor’s approval before switching. In other states, substitution is allowed unless your prescription says “dispense as written.” Always check your state’s rules and ask your pharmacist.
Everyone acts like generics are some conspiracy but the truth is the FDA doesn't regulate fillers and you think that doesn't matter for a drug where 5% more kills you? Wake up.