What Are Biologics for Severe Asthma?
When standard asthma inhalers stop working, doctors turn to biologics-medicines made from living cells that target specific parts of the immune system. Unlike regular asthma drugs that calm inflammation broadly, biologics act like precision missiles. They zero in on one culprit: either IgE, the antibody that triggers allergic reactions, or IL-5, the protein that fuels eosinophil inflammation. These aren’t new ideas, but they’ve changed how people live with severe asthma today.
Omalizumab, the first anti-IgE biologic, hit the market in 2003. Since then, drugs like mepolizumab, benralizumab, and reslizumab-anti-IL-5 therapies-have joined the list. These aren’t cures. They’re add-ons. You still need your inhalers. But for many, biologics mean fewer ER visits, less steroid use, and the ability to breathe without fear.
How Anti-IgE (Omalizumab) Works
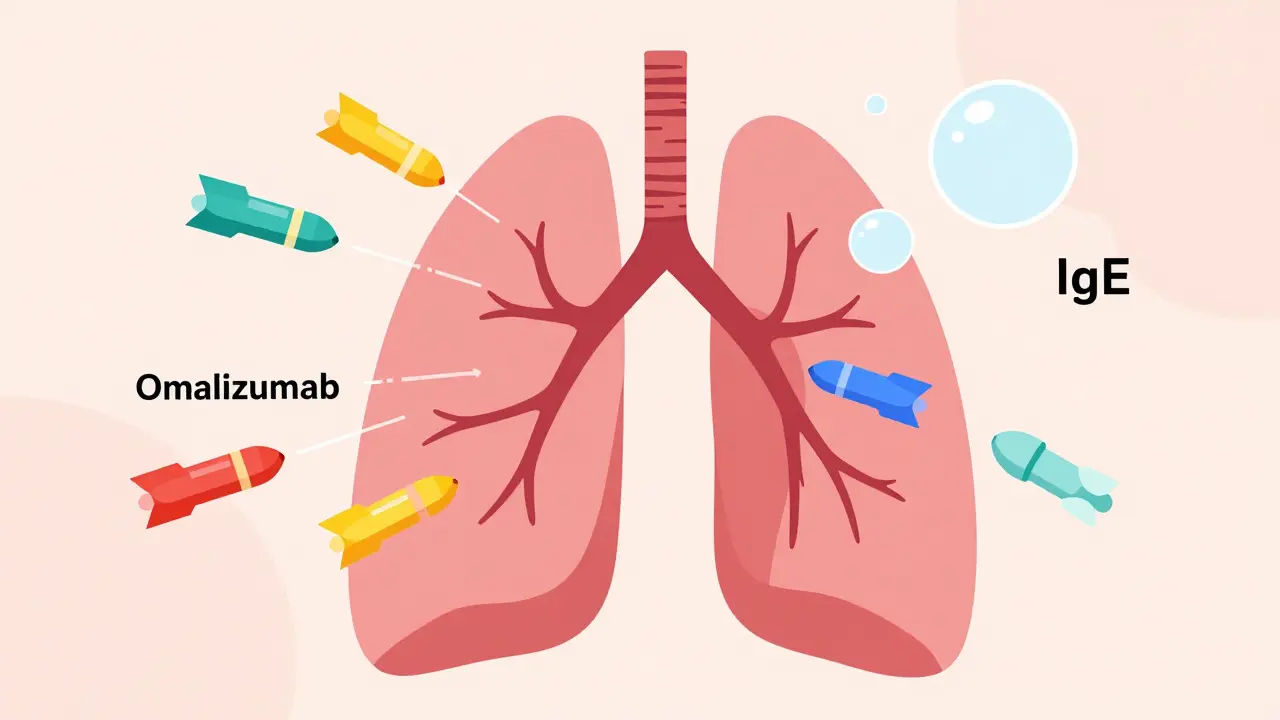
Think of IgE as the alarm system in allergic asthma. When you breathe in pollen, dust, or pet dander, your body overreacts and releases IgE. That triggers mast cells and basophils to dump histamine and other inflammatory chemicals into your airways. That’s when wheezing, coughing, and tightness start.
Omalizumab blocks IgE before it can bind to those cells. No IgE binding = no alarm = no flare-up. It’s only for people with confirmed allergic asthma. That means a positive skin test or blood test showing you’re sensitive to at least one common allergen, like house dust mites or cats. Your total IgE blood level must also be between 30 and 1500 IU/mL. If you don’t meet those criteria, omalizumab won’t work.
It’s given as a shot under the skin every 2 to 4 weeks, based on your weight and IgE level. Most people start noticing fewer symptoms after 12 to 16 weeks. The INNOVATE trial showed a 50% drop in asthma attacks for those who responded. But here’s the catch: if you’re having a flare-up right now, omalizumab won’t help you get through it. It’s preventive, not rescue.
How Anti-IL-5 Therapies (Mepolizumab, Benralizumab, Reslizumab) Work
Not all severe asthma is allergic. Some is driven by eosinophils-white blood cells that swarm the lungs and cause swelling. When these cells stick around too long, they damage airways and make asthma worse. That’s where anti-IL-5 drugs come in.
IL-5 is the signal that tells eosinophils to multiply and survive. Mepolizumab and reslizumab lock onto IL-5 itself, so it can’t reach the cells. Benralizumab goes one step further: it attaches to the IL-5 receptor on eosinophils and literally kills them. Within 24 hours, your blood eosinophil count can drop by 95%. That’s faster and deeper than any other anti-IL-5 drug.
These are for people with eosinophilic asthma. Your doctor checks your blood eosinophil count. If it’s 150 cells/μL or higher in the past year-or 300 or more-you’re likely a candidate. Mepolizumab and reslizumab are given every 4 weeks. Reslizumab requires an IV infusion, so you need to go to a clinic. Benralizumab starts with shots every 4 weeks, then switches to every 8 weeks after three doses. The MENSA and ZONDA trials showed 50-52% fewer asthma attacks. Many patients cut their oral steroid use in half or more.

Who Gets These Treatments-and Who Doesn’t
Biologics aren’t for everyone with asthma. They’re only for severe cases that don’t respond to high-dose inhaled steroids and long-acting beta agonists. Before you start, your doctor must check three things: Are you using your inhalers correctly? Are you taking them regularly? Have you ruled out other causes like GERD, vocal cord dysfunction, or chronic sinusitis?
Biomarkers are non-negotiable. You can’t guess. You need proof. For omalizumab: IgE level and allergy test results. For anti-IL-5: blood eosinophil count. Fractional exhaled nitric oxide (FeNO) is also used in some clinics to measure airway inflammation. If your numbers don’t match the drug’s target, it won’t work. And that’s not a failure-it’s science.
Real-world studies show 30-40% of people don’t respond to biologics. That’s why they’re not first-line. They’re last-line, precision tools. If your asthma isn’t allergic or eosinophilic, these drugs won’t help. You might waste months and thousands of dollars.
Cost, Access, and Daily Life
These drugs cost between $25,000 and $40,000 a year in the U.S. Insurance requires prior authorization-often taking 2 to 3 weeks. Some patients wait months just to get started. Manufacturer programs help with co-pays, but not everyone qualifies.
Most people learn to self-inject after 2 or 3 supervised sessions. The pens are small, easy to use, and come with step-by-step guides. Injections can sting. About 20-30% get redness or swelling at the site, but that usually fades after a few doses. Headaches, sore throat, and sinus pain are common but mild.
Severe allergic reactions (anaphylaxis) are rare-about 1 in 1,000 doses. But if you’ve had a serious allergic reaction before, your risk goes up to 1 in 100. That’s why the first few doses are given in a clinic, and you’re asked to wait 30 minutes afterward.
Some patients report joint pain or fatigue. One Reddit user stopped benralizumab after three shots because of severe joint pain. Others say it gave them their life back. u/AsthmaWarrior2020 went from 3-4 ER trips a year to zero. That’s the kind of story that makes doctors keep prescribing these drugs.
What to Expect Over Time
Improvement doesn’t happen overnight. Some feel better in 4 weeks. Others need 6 months. You won’t wake up one day breathing perfectly. It’s gradual. You’ll notice fewer nighttime symptoms. Less reliance on rescue inhalers. Fewer steroid bursts. Better sleep. More energy.
Doctors don’t stop biologics after a year. Most patients stay on them long-term. Stopping too soon can trigger a rebound flare. The European Respiratory Society says biologics reduce the need for oral steroids by 65% in responders. That’s huge. Long-term steroid use causes bone loss, weight gain, diabetes, and mood swings. Cutting that out is life-changing.
Studies show 78% of users report better quality of life. That’s not just fewer attacks-it’s going back to work, playing with kids, traveling without fear. One woman in Wellington told her doctor she hadn’t missed a single workday in 18 months. That’s what biologics can do.
The Future of Asthma Treatment
Tezepelumab, approved in 2021, is different. It blocks TSLP, an early warning signal from lung cells that kicks off multiple inflammation pathways. That means it works even if you don’t have high IgE or eosinophils. It’s the first biologic that might help non-eosinophilic severe asthma.
Next up: twice-yearly injections. Right now, you’re getting shots every 2 to 8 weeks. New formulations in phase 3 trials could cut that to just two doses a year. That’s a game-changer for adherence.
Researchers are also building algorithms that combine IgE, eosinophils, FeNO, and even genetics to predict who will respond. Right now, we’re guessing. Soon, we’ll know.
The market for these drugs is exploding-projected to hit $12.7 billion by 2028. But access is still unequal. In North America, 2.1% of severe asthma patients get biologics. In Asia, it’s 0.7%. Cost and lack of specialist access are the biggest barriers.
Final Thoughts
Biologics aren’t magic. They’re not for everyone. But for the right person, they’re the difference between living with asthma and living beyond it. If you’ve been stuck on high-dose steroids, frequent ER visits, or feeling like your lungs are always on fire-ask your allergist about testing. Biomarkers matter. Timing matters. And sometimes, the next step isn’t a stronger inhaler. It’s a targeted shot that stops the inflammation at its source.
9 Comments
Write a comment