Tag: biological products
The Purple Book: Understanding Biosimilars and Interchangeability from the FDA
- Benjamin Aghaki-Allen
- Health
- 15 comment
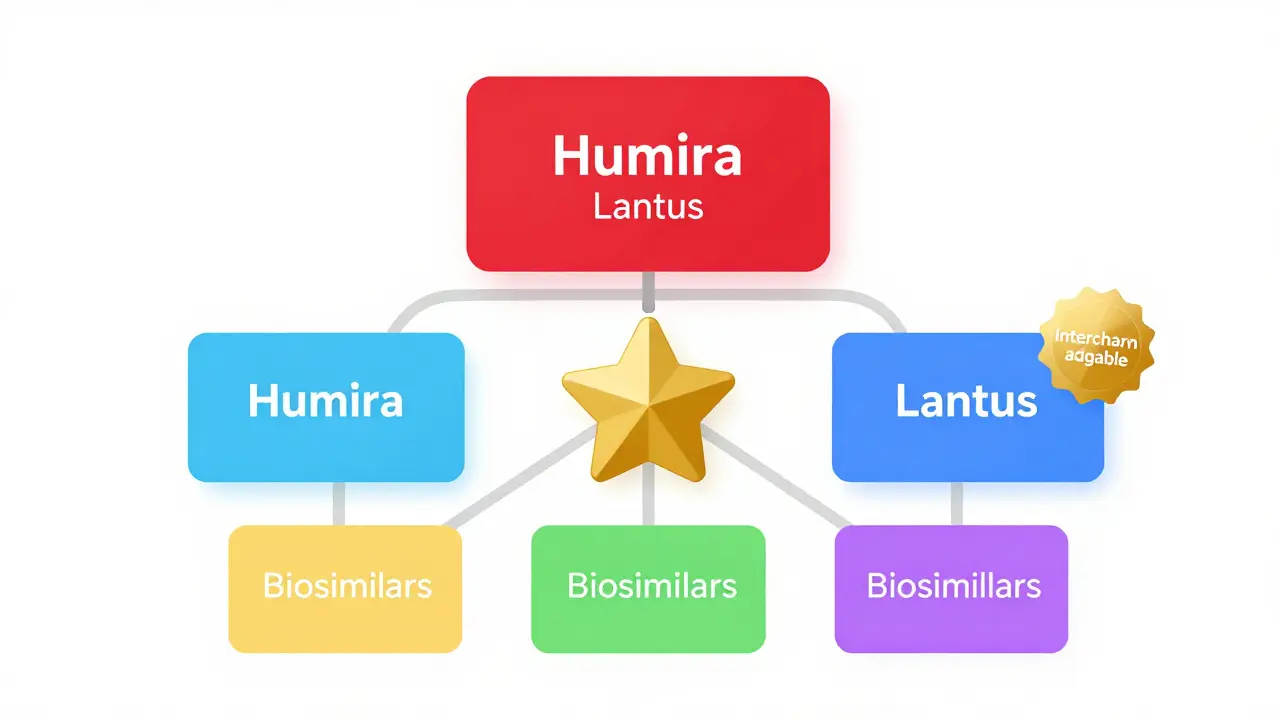
The FDA's Purple Book is the official guide to biosimilars and interchangeable biological products. Learn how it helps patients and pharmacists understand substitution rules, regulatory status, and key differences between biosimilars and interchangeable versions.
VIEW MORECategories
Popular posts
-
Caloric Deficit and Weight Loss: How Energy Balance Really Works
Benjamin Aghaki-Allen -
Doxazosin vs Alternative Blood Pressure Drugs: Benefits, Risks & Best Choices
Benjamin Aghaki-Allen -
Bone-Conduction Hearing Aids: A Practical Alternative for Hearing Loss
Benjamin Aghaki-Allen -
Antibiotics and Myasthenia Gravis: What You Need to Know About Neuromuscular Weakness Risks
Benjamin Aghaki-Allen -
How to Use Dosing Syringes for Kids’ Medicines: Accurate Dosing Guide
Benjamin Aghaki-Allen
Popular tags
- side effects
- medication safety
- online pharmacy
- generic drugs
- medication errors
- drug side effects
- dietary supplement
- gut health
- blood pressure medication
- alternatives
- medication adherence
- Hatch-Waxman Act
- drug interactions
- weight loss
- dosage
- weight management
- quality of life
- cheap generic Zoloft
- affordable sertraline
- Claritin